Imported Cosmetic Registration with CDSCO: Expert Guide to Get Your Cosmetic Product Approved Under Compliances in India
What is CDSCO for Any Cosmetic Importer in India?
To understand CDSCO from importer point of view, Let’s understand the Indian cosmetics market is which is projected to grow at a CAGR of 8.5% from 2021 to 2026, highlighting the importance of regulatory compliance for market entry.
For many importers, the process of getting their cosmetic products approved in India involves several pain points, we are suggesting because we want you (importers) to understand what you are into.Launching cosmetic products in India can be a challenging task for importers. The reason behind this is that they may or may not be aware of the complete process of CDSCO approvals. If something goes wrong in the documentation or compliance handling for CDSCO, the business plan can extend unexpectedly, which no importer wants. For any cosmetic importer, the Central Drugs Standard Control Organization (CDSCO) is the national regulatory authority in India responsible for ensuring that imported cosmetics meet stringent safety and quality standards.
This guide provides insight into CDSCO and its critical role in the registration and compliance process for cosmetic importers in India, no matter where they are situated. These importers can be small-scale businesses to big MNCs, and compliances are mandatory to follow.
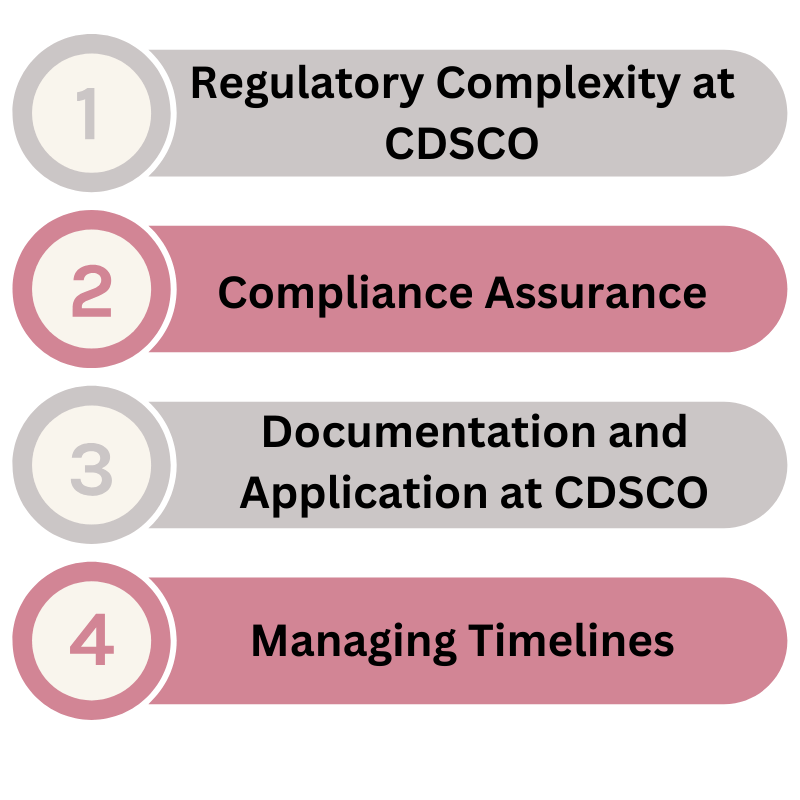
What can be an issue for importer who wants his cosmetic product registered?
- Regulatory Complexity at CDSCO: The intricate and multifaceted requirements of CDSCO can be overwhelming.
- Compliance Assurance: Ensuring that all products adhere to pre-market and post-market compliance standards.
- Documentation and Application at CDSCO: Accurately preparing and submitting necessary documents to avoid rejection.
- Managing Timelines: Effectively handling the approval timeline to ensure timely market entry. Lets take an example you want to launch your cosmetic product after 45 days, you have inventory so you want to launch this in the market as soon as possible. But sometimes process make your plans delayed. This is why you need a CDSCO expert for imported cosmetics registration.
What is the solution for importers facing issues in getting their imported cosmetic products approved?
To address these challenges, it is essential to understand the role of CDSCO and the steps for cosmetics approval required to ensure compliance. Partnering with a pharma regulatory consultant in India can significantly simplify the process, providing expertise and ensuring compliance with all CDSCO requirements. But as an importer you need to know this also because no matter how perfect your service provider becomes his expertise is better useful when you know the basics at least.
Role of CDSCO: The CDSCO, headquartered in Delhi, plays a pivotal role in regulating the importation of cosmetics into India. It oversees:
- Regulatory Compliance: Ensuring that all imported cosmetics meet India’s stringent safety and quality standards.
- Issuance of Licenses: Granting licenses for importing cosmetics, under the Cosmetic Product Registration License.
- Post-Approval Surveillance: Monitoring products post-market to ensure continued compliance with regulations.
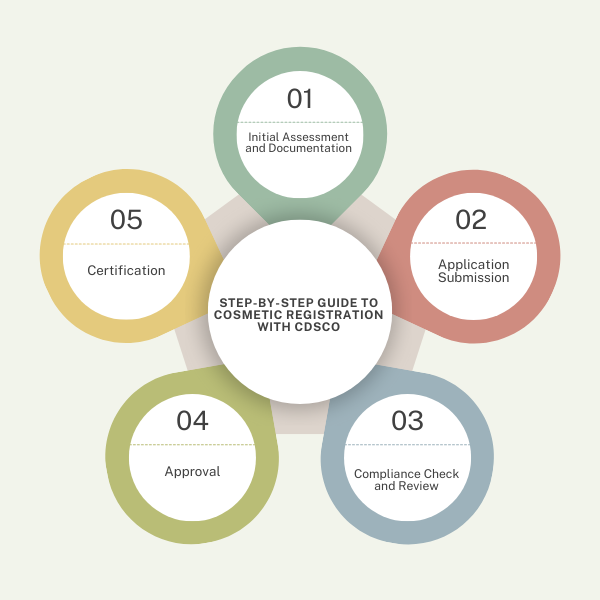
Step-by-Step Guide to Cosmetic Registration with CDSCO:
- Initial Assessment and Documentation:
- Identify Requirements: Verify if your products require CDSCO registration.
- Gather Documents: Prepare all necessary documents, including product information, ingredient details, and GMP certificates.
- Resource Link: CDSCO Documentation Requirements
- Application Submission:
- Forms COS-1 and COS-2: Complete and submit the relevant application forms through the CDSCO portal.
- Resource Link: CDSCO Online Submission Portal
- Compliance Check and Review:
- Pre-Market Compliance: Ensure all product labels comply with the Cosmetics Rules 2020.
- Resource Link: Cosmetic Rules 2020
- Approval and Certification:
- Receive Approval: After reviewing, CDSCO issues the cosmetic registration certificate.
- Maintain Compliance: Continuously update your products to comply with new regulations.
- Resource Link: CDSCO Cosmetic Registration Certificate
Expert Advice and Consultation: Working with a pharma regulatory consultant in India is highly beneficial. These professionals offer expert guidance on application preparation, compliance checks, and post-approval processes, ensuring smooth navigation through the complex regulatory framework.